Why is heavy metals testing important for cosmetic and body care products?
Published: 14th March 2025

Heavy metals such as lead (Pb), arsenic (As), mercury (Hg), cadmium (Cd), chromium (Cr) and Nickel (Ni) are not intentionally added to cosmetics and body care products. However, they may be present as trace contaminants.
Their presence is associated with:
- health risks: leading to skin irritation, neurotoxicity, reproductive issues, and even cancer
- regulatory compliance: many regions have strict limits on heavy metal content in cosmetics
- brand protection: ensures product safety and prevents recalls or legal issues.
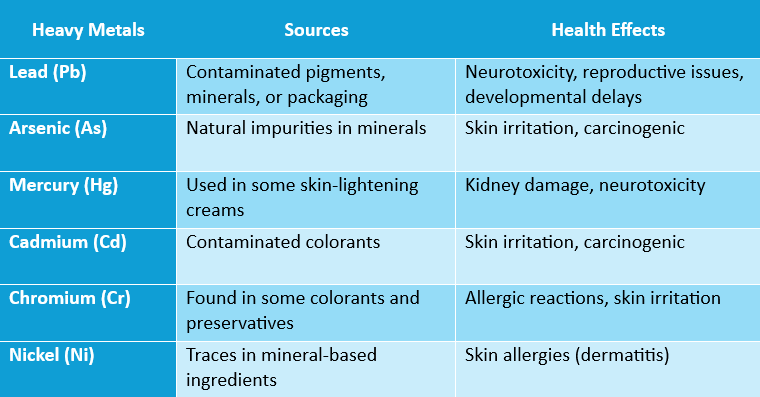
Table 1 shows potential sources and potential health issues if heavy metals are present in cosmetics and body care products.
Due to potential risks associated with heavy metals, the different regulatory agencies worldwide have set limits to ensure consumer safety:
United Kingdom – Post-Brexit Rules (UK Cosmetic Regulations 2020)
- follows EU regulations but requires a separate UK Responsible Person (UK RP)
- cosmetics must be registered on the UK Submit Cosmetic Product Notification (SCPN) portal.
European Union (EU) – Cosmetic Regulation EC 1223/2009
- strict bans on Pb, As, Hg, Cd and restricted use for Cr, Ni. Any heavy metals shouldn’t be intentionally added unless technically unavoidable and present only in trace amounts. EU has a list of over 1,300 banned chemicals (eg hydroquinone, mercury compounds).
- strict pre-market approval: requires a Responsible Person (RP) in the EU for compliance
- must prepare a Product Information File (PIF), including safety assessment, ingredient details, and manufacturing processes
- notification to the EU Cosmetic Products Notification Portal (CPNP) before selling
- banned and restricted substances
- animal testing is banned for finished cosmetics and ingredients.
Canada (Health Canada – Cosmetic Regulations under the Food and Drugs Act)
- lead: ≤10 ppm; arsenic: ≤3 ppm; mercury: ≤1 ppm (except in eye-area cosmetics); cadmium: ≤3 ppm; no specific limit for chromium and nickel
- products must be safe for use but do not require pre-market approval
- mandatory notification within 10 days of first sale via the Cosmetic Notification Form (CNF)
United States (Federal Food, Drug, and Cosmetic Act (FD&C Act) and Fair Packaging and Labelling Act)
- no specific limits but products must be safe for use. In addition, lead levels in cosmetics (recommended ≤10 ppm lead in lip products and externally applied cosmetics). Bans mercury (except in rare cases like eye-area cosmetics at ≤1 ppm).
- cosmetics do not require pre-market approval, but colour additives (except for coal-tar hair dyes) must be FDA-approved
- labelling requirements: must list ingredients, net contents, manufacturer details, and warnings (if applicable)
- claims restrictions: products cannot be marketed as treating or preventing diseases (this would classify them as drugs)
- GMP (Good Manufacturing Practices): while not mandatory is recommended.
India (Bureau of Indian Standards and Drugs and Cometic Act 1940)
- lead: ≤20 ppm; mercury: ≤1 ppm; arsenic & cadmium: banned. No specific limit for chromium and nickel.
- all imported cosmetics need registration with CDSCO (Central Drugs Standard Control Organization).
China (National Medical Products Administration, NMPA)
- lead: ≤10 ppm; arsenic: ≤2 ppm; mercury: ≤1 ppm; cadmium: ≤5 ppm; chromium ≤10ppm; nickel ≤10ppm
- China enforces strict pre-market approval and testing requirements for heavy metals.
Current regulations for cosmetic and body care products have increasingly focused on consumer safety, which has led to more stringent testing for heavy metals. As a result, companies are now required to conduct thorough testing to detect and quantify these contaminants from raw material source to final products and including packaging stage.
Routine testing together with supplier verification and good manufacturing practices can prevent unsafe products from reaching the market, ensuring compliance with legal standards and boosting consumer confidence in cosmetic and body care products.
Sheffield Analytical Services (SAS) can offer testing of heavy metals, ensuring reliable, accurate and compliant results. SAS follows strict test methods and operates with a policy of continuous improvement to ensure it sets the benchmark, investing in industry state-of-art equipment and professional development for its technical personnel.
For the testing of cosmetic and skincare samples SAS follows the principles of BS EN ISO 21392:2021; analytical methods. Measurement of traces of heavy metals in cosmetic finished products using ICP/MS technique. This standard includes a comprehensive guide to analytical methods for measuring traces of heavy metals in cosmetic finished products using the ICP/MS technique and provides reliable and accurate results to ensure the safety and quality of cosmetic and skincare products.
For further information about the full range of Analytical Services we offer, call us on +44 (0)114 231 2121 or email us at analytical@assayoffice.co.uk.
The Sheffield Assay Office was established in 1773, under an Act of Parliament and today the company assays and hallmarks the precious metals - silver, gold, platinum and palladium. Sheffield Assay Office is one of only four UK assay offices who all work to uphold the Hallmarking Act of 1973 and continue to ensure consumer protection for customers purchasing precious metals.
To find out more about the whole range of services offered by Sheffield Assay Office, such as our hallmarking and analytical services, please email us at info@assayoffice.co.uk or complete the contact form on our website at http://www.assayoffice.co.uk/contact-us ,
Sign up here to all the latest news from Sheffield Assay Office direct to your inbox