Explaining XRF and how it has revolutionised precious metal analysis
Published: 19th July 2019

The Sheffield Assay Office has been analysing precious metals for over 240 years, but in the last 15 years this process has been revolutionised by the use of XRF. Our experience in assaying precious metals has been expanded to now include other metals and alloys. We can now offer accurate analysis within an hour, and with no damage to the test item. This article outlines the basic principles of ED-XRF (Energy Dispersive X-Ray Fluorescence), and how we can use it to give fast and accurate results to our customers across a wide range of industries.
What is XRF?
Discovered accidentally by German physicist Wilhelm K. Rontgen in 1895, X-rays are a high energy form of electromagnetic radiation, with wavelengths ranging from 0.01 to 10 nanometres. When an incident x-ray hits an inner shell electron within the atoms of a material, the electron may be ejected. Electrons from a higher shell then drop into the inner shell of the atom to fill the gap, and as a result they give off secondary x-rays with energies characteristic of the elements excited. This process is known as X-ray Fluorescence (XRF).
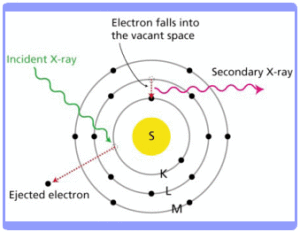
How does the instrument work?
In an energy dispersive XRF instrument, primary x-rays are generated within the x-ray tube, in which a cathode is heated until it emits electrons which are then accelerated by applying a high voltage. These electrons strike the anode material of the tube (typically made of tungsten) which then creates the x-radiation. A filter made of nickel or aluminium is used to optimise the energy distribution of the primary x-radiation for the particular measurement application.
A collimator (aperture) is used to restrict the size of the x-ray beam, which can create a measurement spot as small as 0.3mm. This is ideally suited for testing metal wire, small components of jewellery or thin seams of solder.
A detector measures the energy distribution of the x-ray fluorescence radiation emitted by the sample, which is depicted as a signal spectrum. The characteristic x-rays are labelled K, L, M or N to denote the shell they originated from. X-rays incident upon the front contact area of the detector are absorbed in the bulk silicon region and generate electron-hole pairs. The quantity of charged carriers generated depends on the energy of the incident X-ray. A pre-established electric field between the front contact and the anode causes these electrons and holes to drift along the field lines; i.e. toward the anode. The charge accumulated at the anode is then converted to a voltage by a pre-amplifier. The magnitude of this measured voltage corresponds to the energy of the detected X-ray. Modern silicon-drift detectors experience much less electronic noise and can give much higher count rates (>10,000 cps) and better resolution (<160eV) compared to traditional proportional counters or pin diodes. This is particularly useful for short measuring times.
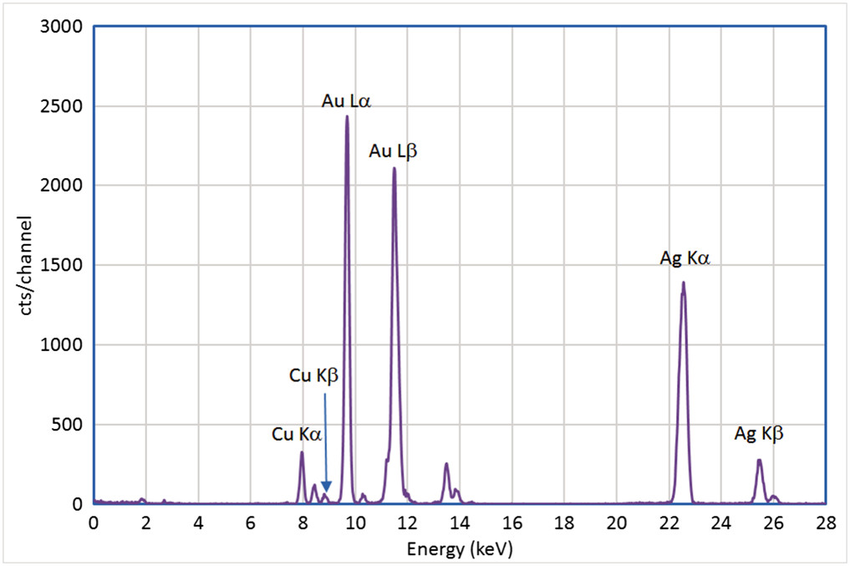
Analysis
The intensity of the spectral peaks depends on the concentration of the particular element within the sample. Semi-quantitative analysis is carried out by the instrument software based on fundamental principles, giving a result in less than a minute.
For quantitative analysis, accuracy is improved by using certified reference materials to calibrate the measurement application. The reference material must closely match the matrix and concentration of the sample. The instrument compares the spectral intensities of the unknown sample to those of the known reference material.
Application
This non-destructive technique combined with experienced analysts and the use of an extensive library of certified reference materials, allows us to confirm that articles of jewellery submitted for hallmarking are up to the required fineness. This helps to protect the public against fraud, and the trader against unfair competition.
Our Sheffield Analytical Services division can use XRF to identify elements of interest in more complex materials such as steels or base metal alloys. We can detect over 90% of the elements in the periodic table, and up to 24 elements simultaneously.
XRF can be used in a cost-effective way across a wide range of applications such as;
- scrap sorting
- archaeological / museum research
- identification of metal and potential contamination
- plating thickness measurement
- quality control
The Sheffield Assay Office was established in 1773, under an Act of Parliament and today the company assays and hallmarks the precious metals - silver, gold, platinum and palladium. Sheffield Assay Office is one of only four UK assay offices who all work to uphold the Hallmarking Act of 1973 and continue to ensure consumer protection for customers purchasing precious metals.
To find out more about the whole range of services offered by Sheffield Assay Office, such as our hallmarking and analytical services, please email us at info@assayoffice.co.uk or complete the contact form on our website at http://www.assayoffice.co.uk/contact-us ,
Sign up here to all the latest news from Sheffield Assay Office direct to your inbox